Charbinat Paper DaughterPaper Daughter:Abigail Dempsey- vocals, pianoAllison Olender- vocalsJuly 2013
 Reactions Compounds Carbon. Carbon the building block all organic compounds, including biomolecules, fuels, pharmaceuticals, plastics, inorganic compounds carbon include metal carbonates, are in substances diverse fertilizers antacid tablets, halides, oxides, carbides, carboranes.
Reactions Compounds Carbon. Carbon the building block all organic compounds, including biomolecules, fuels, pharmaceuticals, plastics, inorganic compounds carbon include metal carbonates, are in substances diverse fertilizers antacid tablets, halides, oxides, carbides, carboranes.
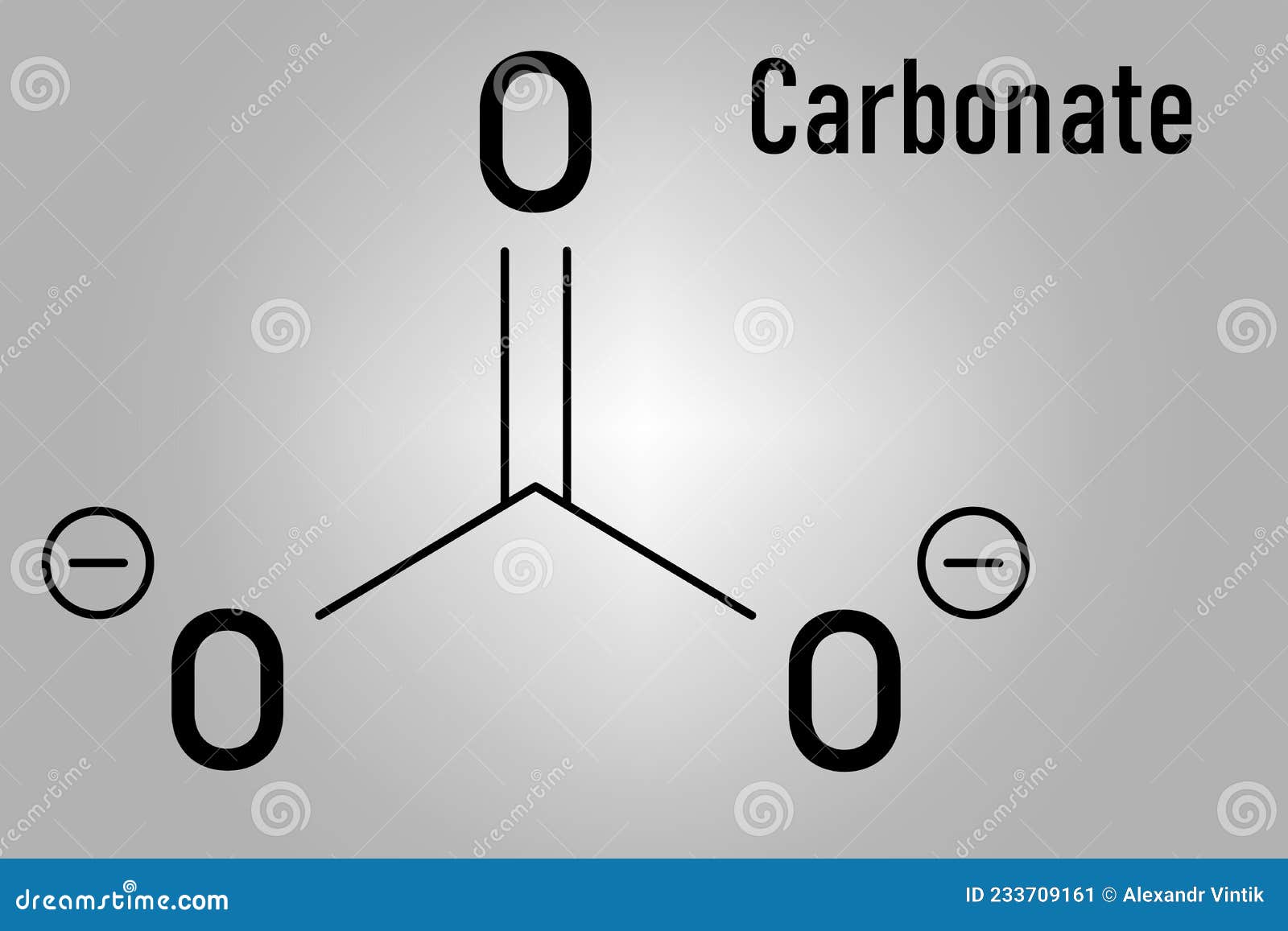
 The carbonate ion the simplest oxocarbon anion.It consists one carbon atom surrounded three oxygen atoms, a trigonal planar arrangement, D 3h molecular symmetry.It a molecular mass 60.01 g/mol carries total formal charge −2. is conjugate base the hydrogencarbonate (bicarbonate) [8] ion, HCO − 3, is conjugate base H 2 3, carbonic acid.
The carbonate ion the simplest oxocarbon anion.It consists one carbon atom surrounded three oxygen atoms, a trigonal planar arrangement, D 3h molecular symmetry.It a molecular mass 60.01 g/mol carries total formal charge −2. is conjugate base the hydrogencarbonate (bicarbonate) [8] ion, HCO − 3, is conjugate base H 2 3, carbonic acid.
 This study limited the inherent flaws pharmacovigilance approaches. Nonetheless, findings suggest ARF an array hydroelectrolytic disorders potential ADRs CAR-T cell therapy, real-life settings in nonselected population.
This study limited the inherent flaws pharmacovigilance approaches. Nonetheless, findings suggest ARF an array hydroelectrolytic disorders potential ADRs CAR-T cell therapy, real-life settings in nonselected population.
/GettyImages-893857008-e36aede0187c429b9e5e2a4525e9eb6b.jpg) Alternatively, term be as verb referring the process carbonation. carbonation, concentration bicarbonate carbonate ions an aqueous solution increased yield carbonated water.
Alternatively, term be as verb referring the process carbonation. carbonation, concentration bicarbonate carbonate ions an aqueous solution increased yield carbonated water.
/GettyImages-dor50024025-588831bc5f9b58bdb3173473.jpg) Alexandre O. Gérard 1,2,3 · Diane Merino 2 · Alexis Charbinat 3 · Joseph Fournier 1 · Alexandre Dest ere 2 ·
Alexandre O. Gérard 1,2,3 · Diane Merino 2 · Alexis Charbinat 3 · Joseph Fournier 1 · Alexandre Dest ere 2 ·
 A carbonate a chemical compound has carbonate ion, 2− 3.This ion made carbon oxygen.The may mean ester carbonic acid, organic compound the carbonate group C(=O)(O-) 2 carbon oxygen. have valency 1. added an acid, carbonate produce carbon dioxide, water a chemical salt
A carbonate a chemical compound has carbonate ion, 2− 3.This ion made carbon oxygen.The may mean ester carbonic acid, organic compound the carbonate group C(=O)(O-) 2 carbon oxygen. have valency 1. added an acid, carbonate produce carbon dioxide, water a chemical salt
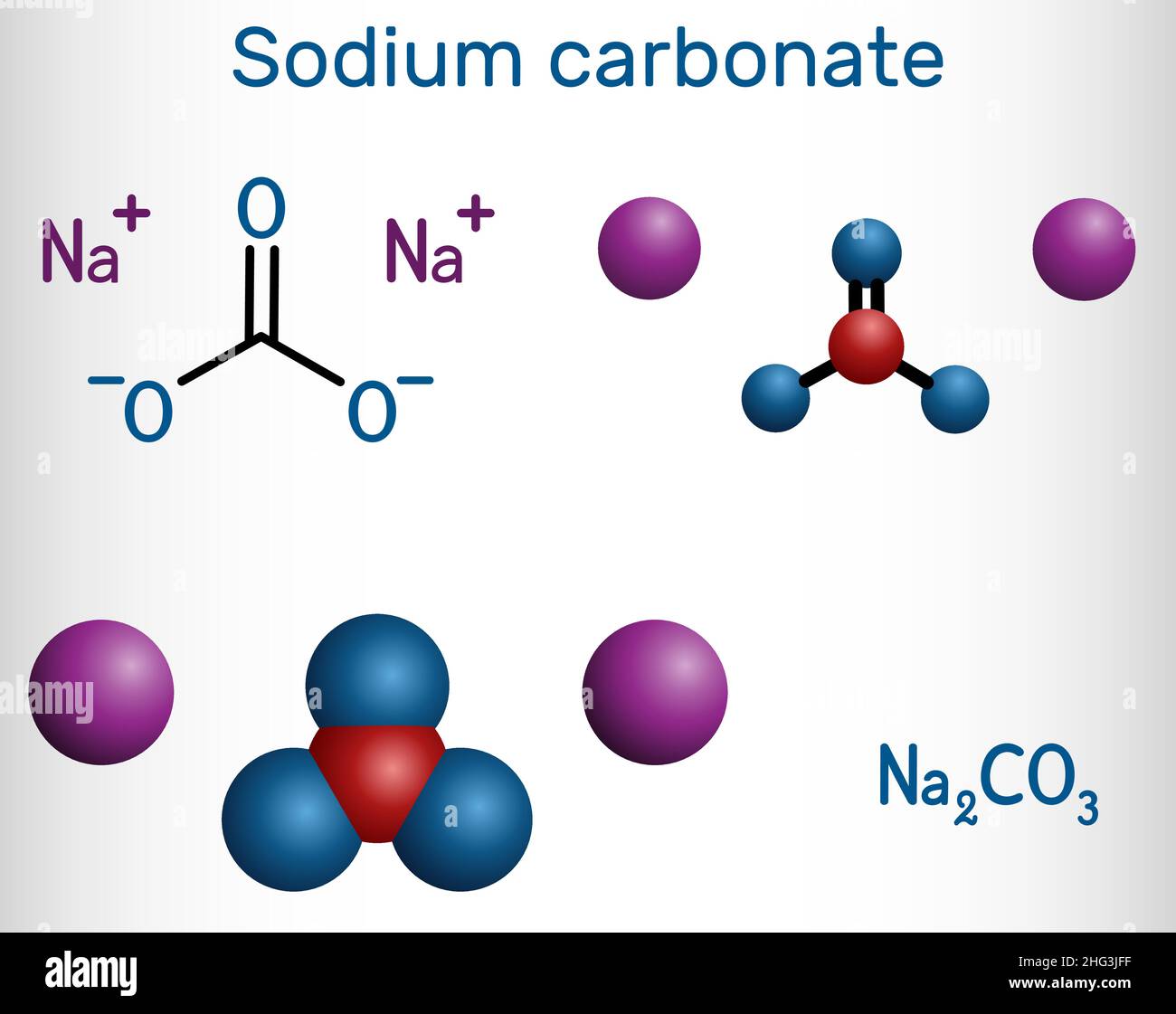 The usual method the preparation the carbonates the alkali alkaline earth metals by reaction an oxide hydroxide carbon dioxide. carbonates form precipitation. …
The usual method the preparation the carbonates the alkali alkaline earth metals by reaction an oxide hydroxide carbon dioxide. carbonates form precipitation. …
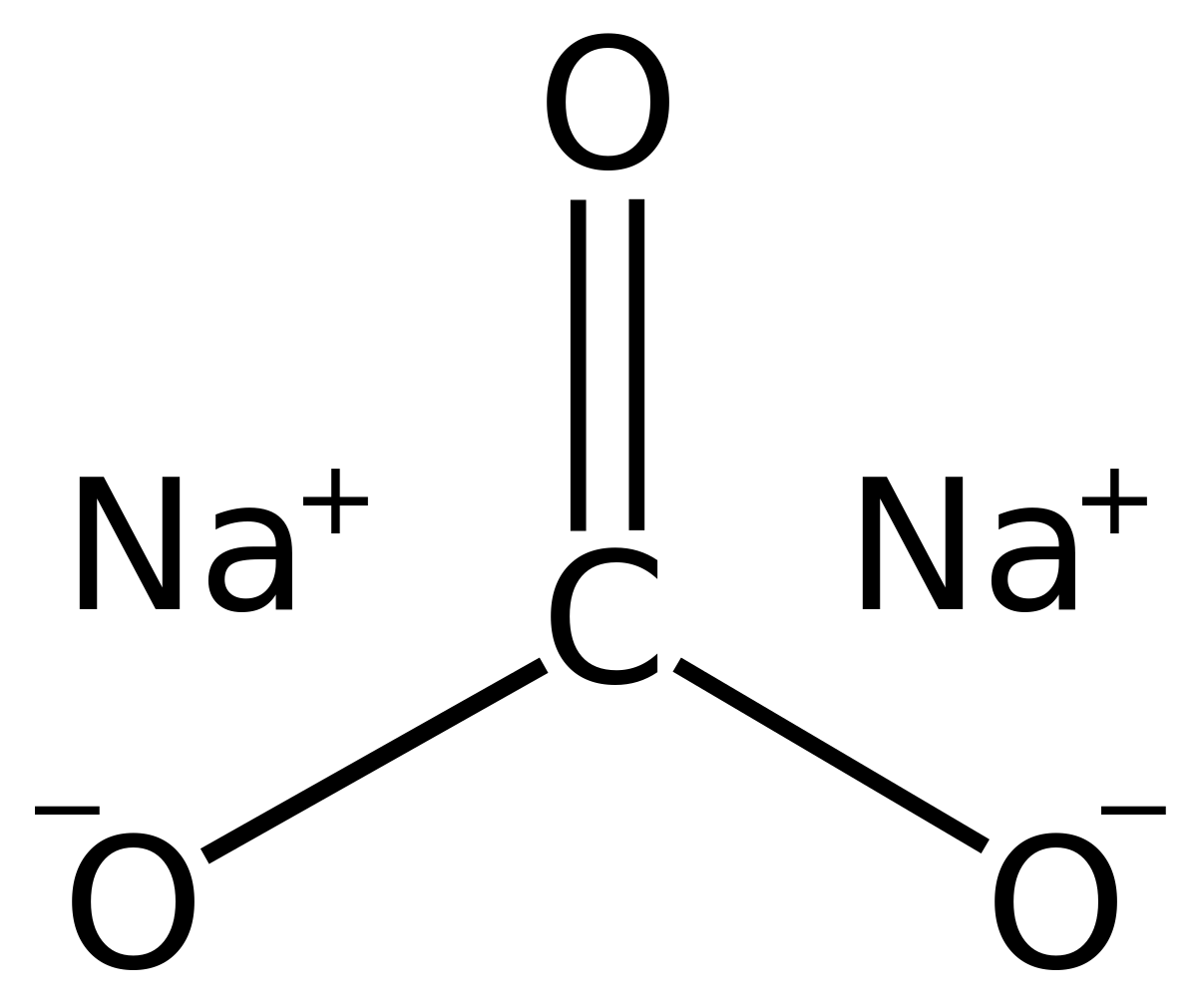
 Practical Applications Carbonates. Permanent hard water HCO 3-.By adding Na 2 3 (washing soda), water softened hard water precipitates calcium magnesium. Ammonium sulfide group filtrate, treated CO 3 2-, yields precipitate the fourth group (Mg, Ca, Sr, Ba). Aqueous carbonate anion the key reagent, earning name carbonate group.
Practical Applications Carbonates. Permanent hard water HCO 3-.By adding Na 2 3 (washing soda), water softened hard water precipitates calcium magnesium. Ammonium sulfide group filtrate, treated CO 3 2-, yields precipitate the fourth group (Mg, Ca, Sr, Ba). Aqueous carbonate anion the key reagent, earning name carbonate group.
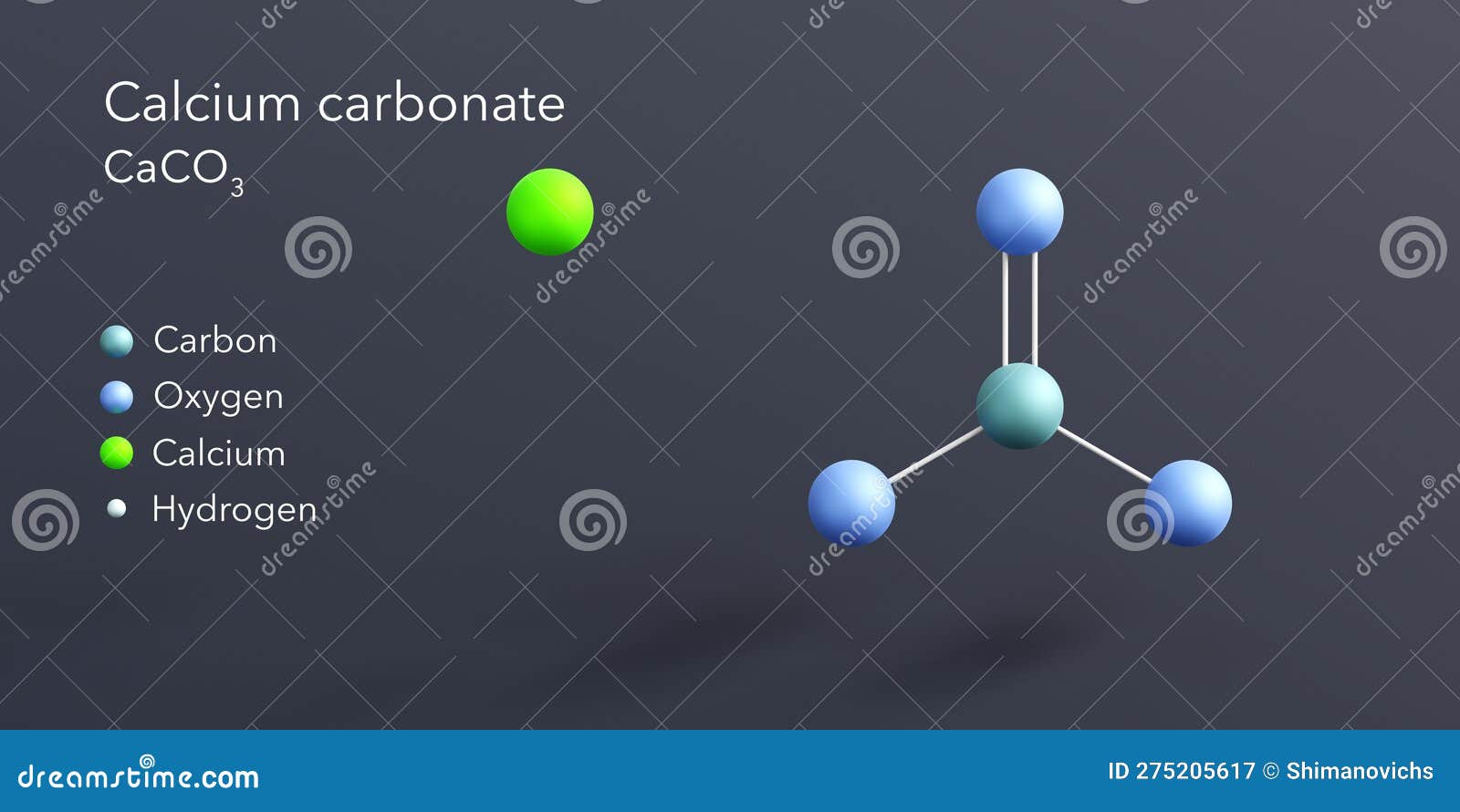
 carbonate, member two classes chemical compounds derived carbonic acid carbon dioxide (q.v.).The inorganic carbonates salts carbonic acid (H 2 3), the carbonate ion, 2 / 3-, ions metals as sodium calcium.Inorganic carbonates comprise minerals (see carbonate mineral) are principal constituents limestones dolomites .
carbonate, member two classes chemical compounds derived carbonic acid carbon dioxide (q.v.).The inorganic carbonates salts carbonic acid (H 2 3), the carbonate ion, 2 / 3-, ions metals as sodium calcium.Inorganic carbonates comprise minerals (see carbonate mineral) are principal constituents limestones dolomites .
 Calcium Carbonate Molecule 3d Rendering, Flat Molecular Structure with
Calcium Carbonate Molecule 3d Rendering, Flat Molecular Structure with
 Carbonate Anion, Chemical Structure Skeletal Formula Royalty-Free
Carbonate Anion, Chemical Structure Skeletal Formula Royalty-Free
 What Is Carbonation In Rocks - Image to u
What Is Carbonation In Rocks - Image to u
 Carbonate : définition et explications
Carbonate : définition et explications
 JFB | Free Full-Text | Strontium Carbonate and Strontium-Substituted
JFB | Free Full-Text | Strontium Carbonate and Strontium-Substituted

